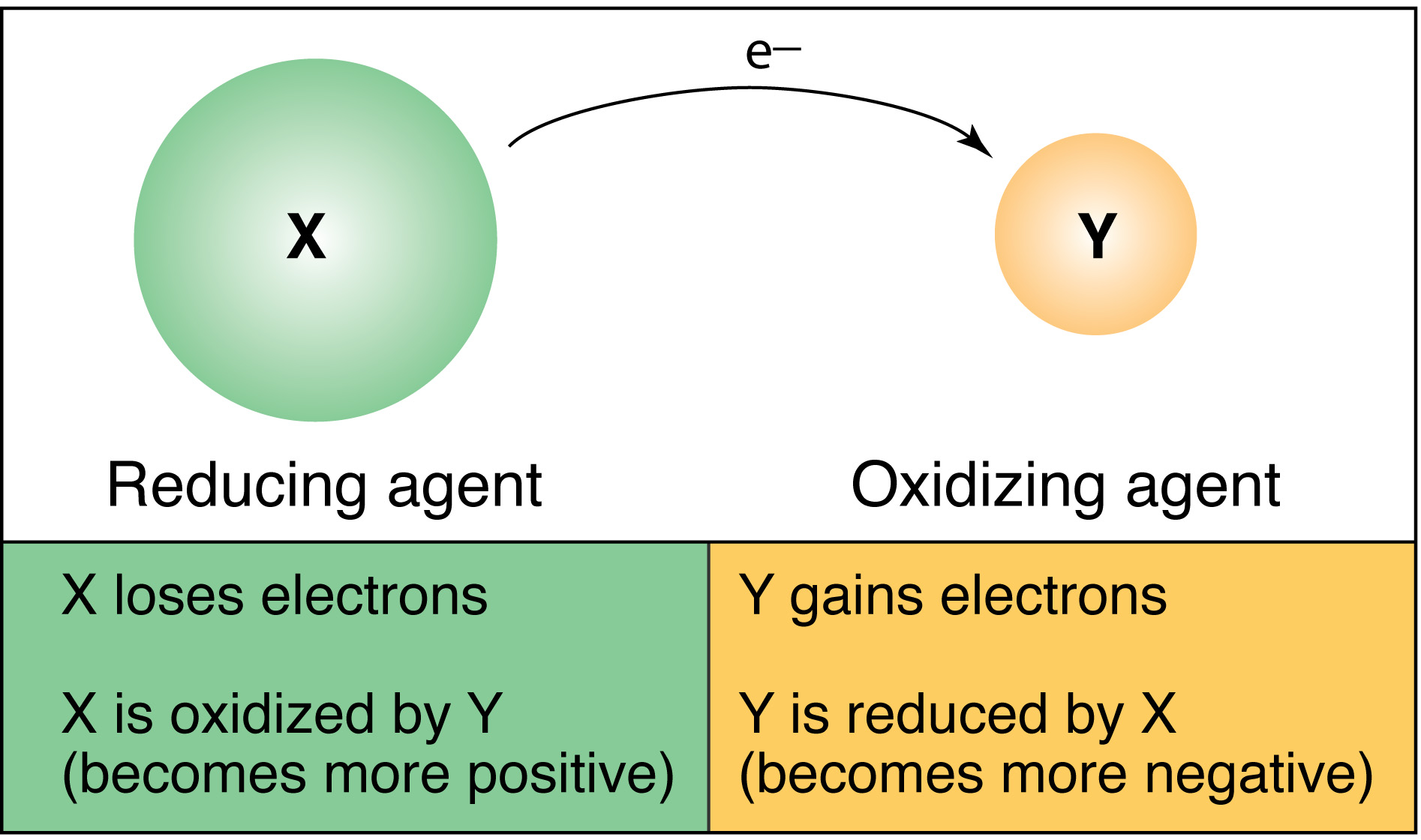
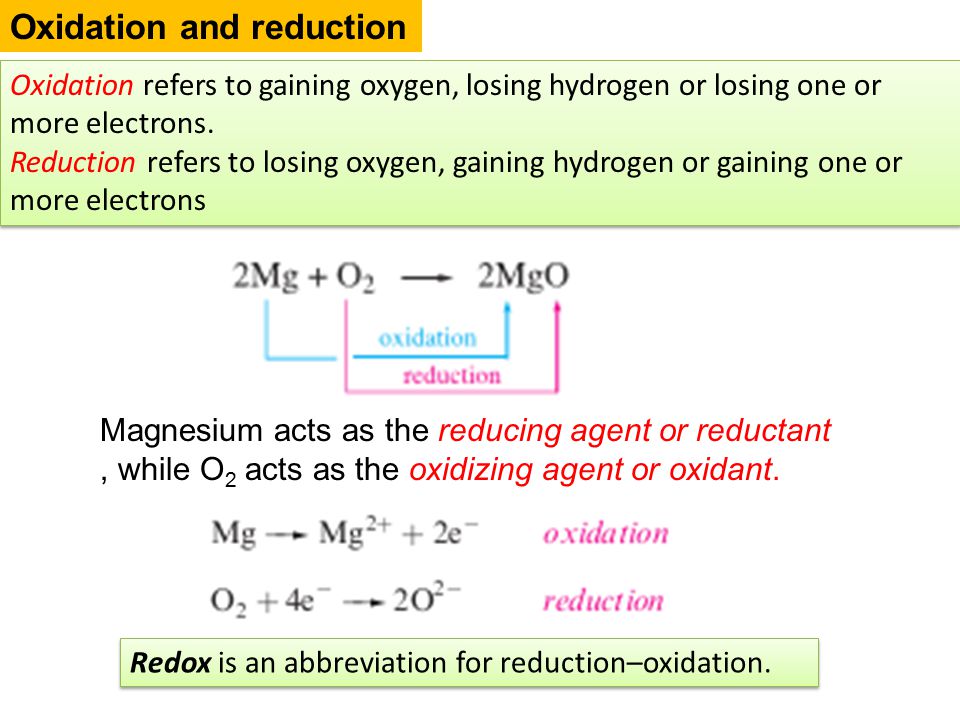
Describe Reduction and Oxidation Potential and How They Are Used
Upon initial reduction Fe species are carburized to Fe 3 C and then to Fe 5 C 2. Recently Mayer and colleagues have reported a comparison between various iron porphyrins for O 2 reduction either in homogeneous solutions or confined at an electrode surface.
The potential cycling tests involved 10000 potential cycles between 0 and 10 V or between 0 and 1.

. Here we reveal key steps for the promotion of this reaction by water when tuning the selectivity of a well-defined CeO 2 Cu 2 OCu111 catalyst from carbon monoxide and carbon dioxide to methanol under a reaction environment with methane oxygen and water. Corrosion engineering is the field dedicated to controlling and preventing corrosion. Corrosion is a natural process that converts a refined metal into a more chemically stable oxideIt is the gradual destruction of materials usually a metal by chemical or electrochemical reaction with their environment.
To describe the adsorption strength of O 2 O. Im a true chemistry freelancer and Subject Matter Expert SME. After they were purged with Ar 99998 and sealed planetary ball milling Fritsch Pulverisette six classic line was carried out at 250 rpm for 15 min followed by a 5.
38 For surface modification they used three different methods. 1 immersion of the catalyst in a solution containing Nafion and thus coating onto a GC electrode 2 immersion of the catalyst. Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential as a measurable and quantitative phenomenon and identifiable chemical change with either electrical potential as an outcome of a particular chemical change or vice versaThese reactions involve electrons moving between electrodes via an electronically.
In the most common use of the word this means electrochemical oxidation of. Identifying the dynamic structure of heterogeneous catalysts is crucial for the rational design of new ones. I bring thirty-two years of full-time classroom chemistry teaching experience and tens of thousands of hours of one-on-one chemistry tutoring across the globe to a seventeen year writing career that includes several best-selling international award-winning chemistry books and a burgeoning.
In this contribution the structural evolution of Fe0 catalysts during CO 2 hydrogenation to hydrocarbons has been investigated by using several quasi in situ techniques. Browse the archive of articles on Nature Chemistry. Highly selective oxidation of methane to methanol has long been challenging in catalysis.
A wide variety of covalent organic cages and two- and three-dimensional covalent organic frameworks have been obtained through dynamic covalent.

Oxidation And Reduction Reactions Basic Introduction Youtube

0 Response to "Describe Reduction and Oxidation Potential and How They Are Used"
Post a Comment